Introduction-
While working in lab, we need to prepare different solutions of different concentration. Dilution is defined as the process of decreasing the concentration of solute in solution by adding more solvent. While diluting the solution, the solute concentration remains the same and volume of solution is increased hence decreasing the concentration. Where as, the concentrated solution is prepared by increasing the concentration of solute. In order to prepare working solution from stock solution, we need to have clear concept of dilution and concentration. So, let’s understand the concept of dilution and its impact on concentration with some sample problems.
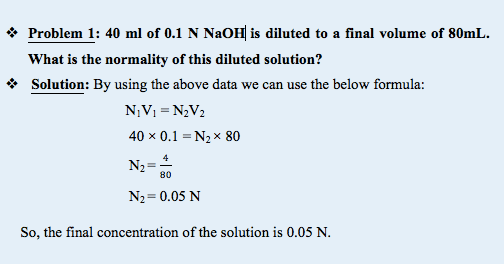

In the next sample problem we will decrease the volume of solute then derive the final concentration –

What happens when two acids of different concentration are mixed?
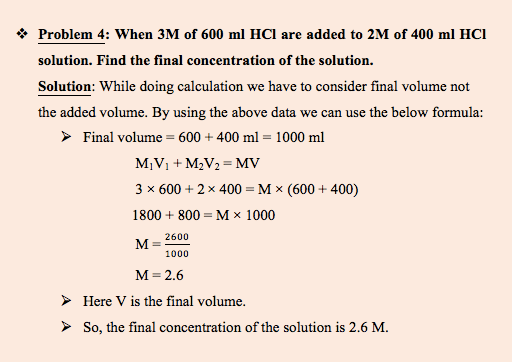
In this sample problem we will take an example of mixing of same acid.
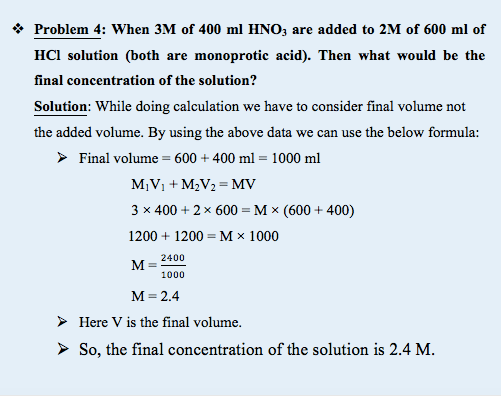
What happens if two different acids of two different concentrations are mixed?
Acids can be monoprotic or polyprotic and hence we need different formula for the calculation.
Monoprotic acids: These acids donate release only one proton or hydrogen per molecule to an aqueous solution and have one equivalence point. Example – Hydrochloric Acid (HCl), Nitric Acid (HNO3) and Acetic Acid (CH3COOH).
We will take example of mixing both types of acids.
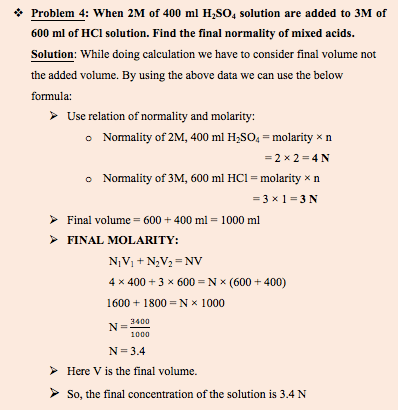
What happens when monoprotic and polyprotic acid are mixed?
Polyprotic acid: These acids can release more than one proton or hydrogen per molecule to an aqueous solution and have one or more equivalence point.
In order to understand such Lab calculation, we need to learn the relationship between normality and molarity.
Relationship between molarity and normality is given as below:
Normality = Molarity × n
Where
n = acidity or basicity depends on the H+ or OH– ion released.
Summary of Learned formulas –
- N1V1 = N2V2
- M1V1 = M2V2
- N1V1 + N2V2 = NV
- M1V1 + M2V2 = MV
- Normality = molarity × n
References –
https://www.chemicool.com/definition/dilution.html


Thanks for this very helpful information