Introduction to TLC –
Thin Layer Chromatography (TLC) is one of the chromatographic techniques used for isolation, identification and quantification of molecules from complex mixtures. The technique was developed in 20th century and it is still getting evolved with the advancement of technology. It has various application in the field of Chemistry and Biology. This article provides a comprehensive overview of Thin Layer Chromatography where we discuss its principle, methodology and applications.
Principle of TLC –
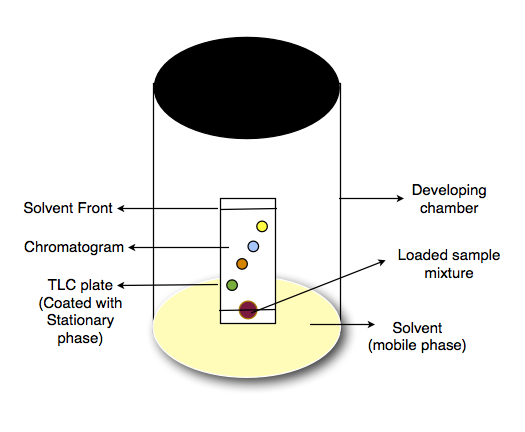
TLC works on the principle of difference in adsorption and capillary action of different molecules present within complex mixtures. In this technique, the sample is applied onto a thin layer of stationary phase (like alumina or silica gel) which is adhered on flat and inert support like glass, plastic or aluminium plate. Stationary phase with loaded sample is then placed in a suitable solvent called mobile phase. The solvent migrate up the thin stationary phase plate due to capillary action. As the mobile phase runs, the compounds present in the sample mixture interact differently with stationary this is because of their different chemical properties (polarities and affinities towards stationary and mobile phase). This results in separation of molecules from sample mixture which can further be quantified.
Materials and Equipments –
- TLC plate – It is flat and rectangular in shape. It mostly made from glass, plastic, or aluminium. This plate acts as supporting material for stationary phase.
- Developing chamber – It is air tight container that can hold the plate vertically. This chamber plays vital role in creative favourable and suitable environment for developing chromatogram.
- Solvent – It is the mobile phase that carries the sample up the TLC plate. The choice of solvent depends up on chemical nature of sample and its constituent ingredients.
- Sample – It is mixture of compounds that need to be analysed.
- Spotting capillary or pipette – It is used for loading the sample on TLC plate.
- Visualisation tools or chemicals – Depending upon the chemical nature of sample we could use UV lamp, iodine vapour chamber or chemical reagents.
Protocol –
TLC plate preparation –
- Weigh desired amount of stationary phase material like silica or alumina powder.
- Mix the stationary phase material with suitable solvent, mix thoroughly and form thick slurry or paste.
- Coat this slurry on TLC plate by applying it at the one end of the plate and spread the slurry across the plate. Make sure that there no bubble or crack formation.
- The layer should be thin and uniform.
- Allow the stationary phase to dry. Before using the plate, the stationary phase need to be activated by gently heating it in oven for 30 min around 100°C.
Sample Application –
- The sample is applied using pipette or capillary tube on TLC plate.
- Draw the baseline.
- It is applied in the form of small spot.
- It is applied at the base of the plate providing sufficient runway for sample to run.
Development –
- TLC plate is placed in the developing chamber.
- Pour the suitable solvent into the chamber such that it is below the level of loaded sample.
- Enclose the chamber with its lid.
- The vapours of the solvent get saturated in the closed chamber creating suitable environment for running the sample on to the TLC.
- Due to capillary action, the solvent rises through TLC plate. While moving, it takes along the sample compounds with it.
- As the different compounds in the mixture sample have different affinities towards stationary and mobile phase, they interact differently with them and allowing their separation.
Visualisation –
- After sufficient time (around 30 min), remove the TLC plate from chamber and mark the highest point reached by the solvent. This highest point is called solvent front.
- If the separated compounds are UV active compounds then we can use UV lamp. For non-UV active compounds we can use iodine chamber. We can also visualise the separated ingredients of our sample mixture by spraying or dipping it with specific chemical agent.
- Visualisation steps results in the appearance of colored spots.
Analysis –
- Measure the distance travelled by each compound from baseline to its respective spot.
- Calculate the retention factor for each compound. Retention factor or Rf value is the ratio of distance traveled by solvent front by distance traveled by the components or molecule.
- Rf value is specific to particular compound and hence can be used for identifying the unknown compound by comparing it with standards.
Documentation –
- Take the photographs and other relevant details and note it in your lab-book.
Advanced TLC –
With research the development, Thin Layer Chromatography is getting evolved. Following are the major notable advancements –
- Principle and technology of High Pressure chromatography is employed in Thin Layer Chromatography forming HPTLC (High Pressure Thin Layer Chromatography) which enhances the efficiency and accuracy.
- TLC is also been associated with Mass Spectrometry (MS) forming TLC-MS allowing us to explore identification and finding molecular mass at the same time.
- Computer and automated assisted TLC overcomes the manual mistakes and gives error free results.
- With the development of nanotechnology, thin layer chromatography can now also employed for testing and detecting trace level compound.
- Knowing the need of the hour, scientist are upcoming with eco-friendly technology and same is being attempted in the TLC.
Applications of TLC –
- Pharmaceuticals – It is used for testing drug quality and purity. It is also further used for identification and quantification of active drug compounds.
- Material Sciences – It is applied for characterisation of polymers, materials and nanoparticles.
- Life Sciences – It is used for isolation, identification and quantification of isolated molecules from living sample.
- Forensic Science – It is employed of banned drugs and toxins.
- Environment Science – It is used for identification and quantification of pollutants.
- Food Industry – It is used for testing food quality and detecting contaminants.
Future Prospects & Conclusion –
Thin Layer Chromatography is very important tool for isolation, identification and quantification of compounds. It is widely employed in different fields. With the further advancement of technology and methodologies, it seems to be promising analytic tool for scientific research and analytical chemistry. It is easy to use and provides promising results and it is adaptable making it a key technique in the scientific world.
References –
- https://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/chrom/wback3.htm
- https://chem.libretexts.org/Ancillary_Materials/Demos_Techniques_and_Experiments/General_Lab_Techniques/Thin_Layer_Chromatography
- https://www.chemguide.co.uk/analysis/chromatography/thinlayer.html
- https://pubmed.ncbi.nlm.nih.gov/24182936/
Dr. Sangha Bijekar has 9 years of Teaching Experience at University level. She loves to get engage in teaching and learning process. She is into blogging from last two years. She intends to provide student friendly reading material. She is avid Dog Lover and animal rescuer. She is learned Bharatnatyam and Katthak Dancer. She is into biking and She also loves to cook.