‘Pharmacogenomics Determining the right drug in the right dosage at the time for each and every patient’
Introduction –
In Pharmacogenomics we study the role of genome in drug response. Pharmacogenomics is a wide-ranging term, which studies how all of the genes can influence response to drugs, it is to study the variations of DNA and RNA features correlated to drug response. Whereas the term pharmacogenetics is a subdivision of pharmacogenomics which usually refers that how the variation of a single gene affect the response to a single drug. This field is relatively new because it is the combination of pharmacology and genomics. It helps to enhance drug therapy with minimal adverse effects. This type of approaches promise the arrival of “personalized medicine”; where drugs in addition to drug combinations are optimized for each individual.
History –
Pythagoras first recognized the concept of Pharmacogenomics around 510 BC. When he made a simple connection among the dangers of the fava bean ingestion with hemolytic anemia and oxidative stress. Then this documentation was later validated and credited to deficiency of G6PD in the 1950s and it was termed as favism. In case of prolonged paralysis and fatal reactions linked to genetic variants in patients who mainly lacked pseudocholinesterase then the succinylcholine is injected and this was first reported in 1956. Friedrich Vogel of Heidelberg first coined the term pharmacogenetic in 1959.
Twin studies supported the implication of genetic involvement in drug metabolism in the late 1960s, then the identical twins sharing remarkable similarities to drug response compared to the fraternal twins.
The first FDA approval of a pharmacogenetic test for alleles in CYP2D6 and CYP2C19 was in 2005. These and other discoveries that led to the term pharmacogenomics. Today, there are cumulative number of genes that the polymorphisms are identified and that are related to variable drug response. Genome-wide analysis is mainly helping to identify previously unpredictable new genes who are associated with disease and drug response. It helps to enhance drug therapy with minimal adverse effects. This type of approaches promise the arrival of “personalized medicine”; where drugs in addition to drug combinations are optimized for each individual.
Genetic Variants and Drug Response –
Some example of genetic variants that influence drug response are:
- Insertions and deletions, copy number variations.
- Single nucleotide polymorphisms which is a DNA sequence variation occurring when a single nucleotide adenine, thymine, cytosine, or guanine in the genome varies between the members of the biological species or paired chromosomes in a human.
Responses to all the drugs virtually vary between individuals because of some factors:
- Intrinsic factors which means age, health, genetics and etc.
- Extrinsic factors which means the diet, the use of concomitant drugs that may affect drug PK parameters.
- Genes relevant to the drug’s PK.
- Genes that influence ADRs susceptibility.
Drug Metabolizing Enzymes –
There are numerous known genes which are mostly responsible for alterations in drug metabolism and response.
- Cytochrome P450s
- TPMT
- VKORC1
Cytochrome P450s:
The most predominant drug-metabolizing enzymes are the Cytochrome P450 enzymes. The term Cytochrome P450 was devised in 1962 by Omura and Sat
CYP2B6:
CYP2B6 plays a very vital role in the metabolism of drugs as well as the anti-HIV drug efavirenz, the anti-malarial artemisinin, the antidepressants, the anti-cancer drug cyclophosphamide etc. This can be really a polymorphic catalyst with the variant CYP2B6*6.
CYP2D6:
CYP2D6 is the most eminent and widely studied CYP gene. This gene is of an excellent interest due to its extremely polymorphic nature, and its participation within the high range of medication metabolisms.
CYP2C19:
CYP2C19 is that the second most generally studied and a well understood gene in pharmacogenomics. For CYP2C19 over 28 genetic variants have been recognized.
CYP2C9:
CYP2C9 constitutes the majority of the CYP2C subfamily, it is involved in the metabolism of about 10% of all the drugs, which also include the medications with narrow therapeutic windows such as warfarin and tolbutamide.
CYP3A4 & CYP3A5:
The CYP3A family most profusely found in the liver. CYP3A4 accounting 29% of the liver content.
TPMT:
Thiopurine methyltransferase (TPMT) catalyzes the S-methylation of thiopurines, thus regulating the balance between cytotoxic thioguanine nucleotide and inactive metabolites in the hematopoietic cells. TPMT is extremely involved in 6-MP metabolism and TMPT activity and genotype is known to affect the risk of toxicity. Extreme levels of 6-MP may cause myelosuppression and myelotoxicity.
VKORC1:
Vitamin K epoxide reductase complex subunit 1 which is responsible for pharmacodynamics of warfarin. VKORC1 along with CYP2C9 are very much useful for recognizing the risk of bleeding during warfarin administration.
Predictive Prescribing–
Genotypes of the patient are usually classified into the following predicted phenotypes:
- UM- Ultra-rapid metabolizer: the patients with substantially increased metabolic activity.
- EM- Extensive metabolizer: the normal metabolic activity.
- IM- Intermediate metabolizer: the patients with reduced metabolic activity; and
- PM- Poor metabolizer: the patients with little to no functional metabolic activity.
Drugs mainly classified into two groups such as: Active drugs and Prodrugs.
Active drugs refer to drugs that are mainly inactivated during the metabolism, and prodrugs are mainly inactive until they are being metabolized.
Factors Describing When Pharmacogenetics Studies Are Required:
If in vitro and in vivo studies designate that a known functionally polymorphic enzyme is:
- Likely to represent a vital factor in the formation, elimination or distribution of a pharmacologically active metabolite.
- Probably to be important in the metabolism and abolition of the drug.
- In case clinical studies designate those changes in (PK) properties cannot be clarified by other intrinsic or extrinsic factors and are likely to influence the efficacy of the drug.
Factors Describing When Pharmacogenetics Studies Are Recommended:
- If in vitro data indicate that a polymorphic enzyme contributes to the PK properties of the active substance, but that the measureable role is comparatively low based on the in vitro data.
- Or if there is high inter-individual PK variability, or there are PK outliers with higher.
- Or if major PK alterations between ethnic groups.
Factors Describing When Pharmacogenetics Studies Are Considered In Phase III Clinical Trial Studies:
- If difference in dosage is likely to be clinically pertinent.
- If difference in exposure is probably going to be clinically pertinent, but due to the available marketed formulations it’s impossible to regulate the doses. If the patients tested positive for a specific genotype or phenotype then he/she should be omitted from the trials.
- If initial studies suggest that a marked difference in drug exposure related to polymorphic enzymes lacks clinical significance, phase III trial should approve that genotype has no impact on the treatment.
Why Drugs Works Differently In Different People:
Drug activation— In case of cancer the drugs that treat cancer need to be “turned on” so that it work this process is called activation.
Drug deactivation— Drugs also got to be “turned off” to limit the drug’s exposure thanks to healthy tissues this process is named deactivation.
Some of the people may have slower enzymes due to which the chemical reactions takes place very slowly. As a result, high levels of the drug may remain in their bodies for a very long time. Due to this they might have more side effects from the drug.
In addition to pharmacogenomics, other factors may influence a person’s reaction to a drug such as:
- Age and gender.
- The cancer’s stage.
- Smoking and drinking alcohol.
- Medications taken for many other conditions.
Little More Information Related To Pharmacogenetics –
How Providers Use Pharmacogenetics?
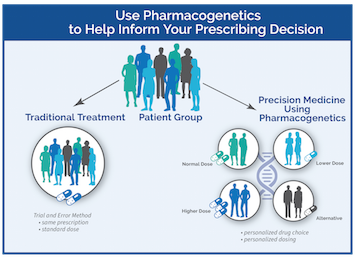
Craft a patient’s medication to their distinctive genetic appearances could at some point replace the one-size-fits-all strategy to the drug choice and dosing that they unremarkably used nowadays.
Pharmacogenetics Today–
Over 250 prescription medications accommodate Pharmacogenetic evidence in their FDA approved labels.
The label’s info contains the identification of biomarkers – the first measurable indicators related to a patient’s specific condition. The labels also identify targeted drug therapy specific for that genetic abnormality as well as list the genetic variations which may influence how a drug is metabolized or diminished within the body and is probably going to cause a major adverse event.
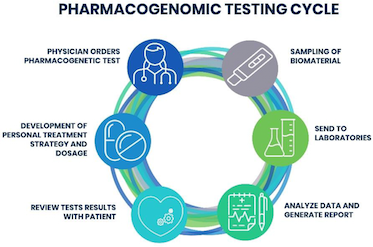
How Does Pharmacogenetics Work?
The cytochrome P450 system are the family of enzymes that are found throughout the body which are liable for the synthesis and metabolism of the various molecules and chemicals within the cell, most remarkably including the active ingredient of most drugs.
Common variations are known as polymorphisms in the genes that determine the cytochrome P450 enzyme activity and may affect the purpose of the enzymes. These are most commonly seen in the breakdown of medication.
If the cytochrome P450 accelerator metabolizes a drug slowly, the drug remains active longer and a lower dose is required to induce the required result whereas usual doses might cause the toxicity.
Additionally, there are other genes outside the cytochrome-p450 system that affect the drug metabolism and as a result patient’s response to the medications.
Now A Question Can Arise That-
How Pharmacogenomics Differs From Genetic Testing?
Standard genetic testing search for specific genes and it also detect abnormalities in genes, chromosome or proteins. Whereas, in Pharmacogenomics, the researchers still acknowledge the cistron variations that have an effect on however a drug works. As the personalized medicine growing, testing for the gene variations may become more common.
Pharmacogenomic Testing In Practice–
Here are certain examples related to pharmacogenomic testing in the cancer care
Colorectal Cancer
Irinotecan is a kind of chemotherapy. Where the doctors normally use it to treat the colon cancer. In certain people, genetic variations cause a deficiency of the enzyme name UGT1A1. This enzyme is mainly responsible for metabolizing the Irinotecan.
Acute lymphoblastic leukemia (ALL)
About 10% of the people have genetic variations in an enzyme termed thiopurine methyltransferase (TPMT). TPMT is mainly responsible for metabolizing the chemotherapy for ALL.
Fluorouracil is a kind of chemotherapy. It is used to treat the several types of cancer including colorectal, breast, pancreatic cancers and etc.
Benefits Of Pharmacogenomics–
Some of the benefits of pharmacogenomics are:
- It improves patient safety. Severe drug reactions cause more than an expected 120,000 hospitalizations in each year. Pharmacogenomics try to prevent all these by identifying patients at risk.
- It improve health care costs and efficiency. Pharmacogenomics help to find appropriate medications and appropriate doses quickly.
Challenges To Pharmacogenomics–
Here are some challenges in the growth and practical use of pharmacogenomics:
- It is expensive, particularly if the insurance does not cover the costs.
- Access to some definite tests might be limited in some of the places.
Pharmacogenetics Approach For The Improvement Of Covid-19 Treatment–
The treatment of coronavirus unwellness 2019 has been a challenge. The effectualness of many medication has been evaluated and variability in drug response has been ascertained. genetics may justify this variation and improve patients’ outcomes with this advanced disease; nonetheless, many disease-related problems should be fastidiously reviewed inside the pharmacogenetic study of COVID-19 treatment. We tend to aimed to elucidate the pharmacogenetic variants rumored for medication used for COVID-19 treatment (remdesivir, oseltamivir, lopinavir, ritonavir, azithromycin, antimalarial drug, anti-inflammatory, ivermectin, and dexamethasone).
Besides, nongenetic factors like drug-drug interactions and inflammation ought to be thought-about inside the design for personalized medical aid of COVID-19.
Applications–
The list below offers commonly known applications of pharmacogenomics:
- Drugs have improve safety and reduce ADRs.
- Tailor treatments to satisfy patients’ unique genetic pre-disposition, identifying optimal dosing, and
- Drug discovery has improved towards targeted human disease.
Pharmacogenomics is pertinent in numerous areas of medicine such as including pain management, cardiology, oncology, psychiatry etc. In forensic medicine also there’s an area, during which pharmacogenomics want to determine the explanation for the death in drug-related deaths where no findings emerge using autopsy.
In the arena of cancer treatment, pharmacogenomics tests are mainly used to recognize which patients are most likely to retort to the certain cancer drugs.
Clinical Implementation–
Initiatives to spur approval by clinicians include ubiquitous pharmacogenomics program in Europe and the clinical pharmacogenetics application consortium in the US, and in 2017 study of European clinicians, in the previous year two-thirds had not ordered the pharmacogenetic test.
The biggest private health insurer, UnitedHealth care, broadcasted that it would pay for genetic testing to forecast the answer to the psychiatric drugs in 2019.
Polypharmacy–
A potential role pharmacogenomics might play would be to scale back the occurrence of polypharmacy. It is theorized that with tailored drug treatments, patients will not have the need to take several medications that are planned to treat the same condition. In doing so, they could possibly minimalize the amount of ADRs, and have enhanced the treatment outcomes, and can save the costs by avoiding purchasing unimportant medications.
Future –
Computational advances have facilitated cheaper and faster sequencing. Research has focused on the combinatorial chemistry, genomic mining, omic technologies and etc.
Development of personalized drug therapies will increase, as the cost per genetic test decreases. It is a large step to carry pharmacogenetic technology into everyday medical decisions.
Ethics –
In the area of bioethics Pharmacogenetics has become a controversial issue. Privacy is the major concerns. The evidence of benefit or the risk from a genetic test might only be suggestive, which could cause dilemmas for the providers. Drug development might be affected, with rare genetic variants generally receiving less research.
References –
- https://www.cdc.gov/genomics/disease/pharma.htm
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2806804/
- https://en.wikipedia.org/wiki/Pharmacogenomics#Predictive_prescribing
- http://www.encepp.eu/publications/documents/6.1_Pharmacogenomics.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2792612/
- https://academic.oup.com/bmb/article/124/1/65/4430783
- https://clinfowiki.org/wiki/index.php/Pharmacogenetics
- https://psychology.wikia.org/wiki/Pharmacogenetics
- https://medlineplus.gov/genetics/understanding/genomicresearch/pharmacogenomic/
- https://www.genelex.com/what-is-pharmacogenetics/
- https://www.bioinformatics.org/wiki/Pharmacogenetics
- https://www.cancer.gov/publications/dictionaries/cancer-terms/def/pharmacogenetics
- https://www.nigms.nih.gov/education/fact-sheets/Pages/pharmacogenomics.aspx
- https://en.wikipedia.org/wiki/Pharmacogenomics
- https://medlineplus.gov/genetics/understanding/genomicresearch/pharmacogenomic/
- https://www.nature.com/articles/537S60a
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1874402/


