Summary-
Gene is a segment of DNA or RNA that encodes for a functional protein. The main objective of plant biotechnology is to create new varieties of cultivated plants by manipulating DNA molecule. Nowadays, Gene transfer methods has become the most versatile and easy to handle protocols for improving crop production and for study or analyse the function of the genes. This article mainly describes the methods used to transfer a gene in plants. The main methods discussed in the below article are gene transfer by Agrobacterium tumefaciens, microprojectile bombardment, electroporation of protoplast, PEG mediated method, microinjection, liposome mediated gene transfer and through ultrasonication method.
Keywords – Gene, Gene transfer, Transformation technology.
Introduction-
Genetic manipulation of plants has been done by plant breeders for years in order to improve the quality of crops and is done with a great success. Many different types of methods have been developed by the breeders for crossing plants in order to transfer and maintain the required and desirable characteristics in inbred lines. The problem with the classical plant breeding is that it is very slow. It generally takes fifteen to sixteen years to produce a new variety. The other drawback is that it is limited to sexually reproducing plants and there is also the possibility of transferring undesired genes.
The limitation of classical plant breeding can be overcome by using R-DNA technology (recombinant DNA technology). This technology enables the plant geneticist to identify and make clones of specific genes for desirable traits. Through this technology the introduction of new genes into plants using different methods is now possible. This article is all about the different methods of gene transfer in plants.
Gene Transfer Methods in Plants –
The methods involved in gene transfer procedure are as follows:
- Agrobacterium tumefaciens mediated gene transfer
- Microprojectile bombardment
- Electroporation of protoplast
- PEG mediated gene transfer
- Microinjection
- Liposome mediated gene transfer
- Pollen tube pathway method
- Ultrasonication methods
Benefits of Gene Transfer –
- Provide resistance against viruses.
- Develop drought resistance plants.
- Herbicide resistance plants can be created.
- Acquire insecticidal resistance property.
- To improve the nutritional quality of plants.
- To make the plants such that they can grow in any seasons.
- Delayed ripening can be done.
Agrobacterium tumefaciens mediated gene transfer–
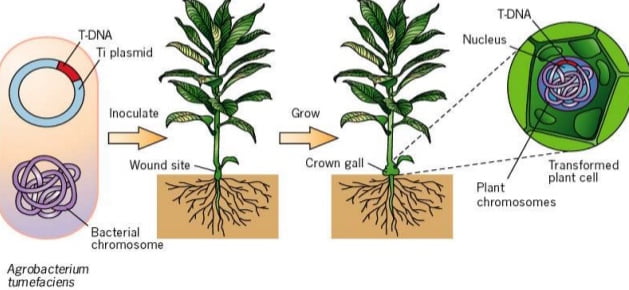
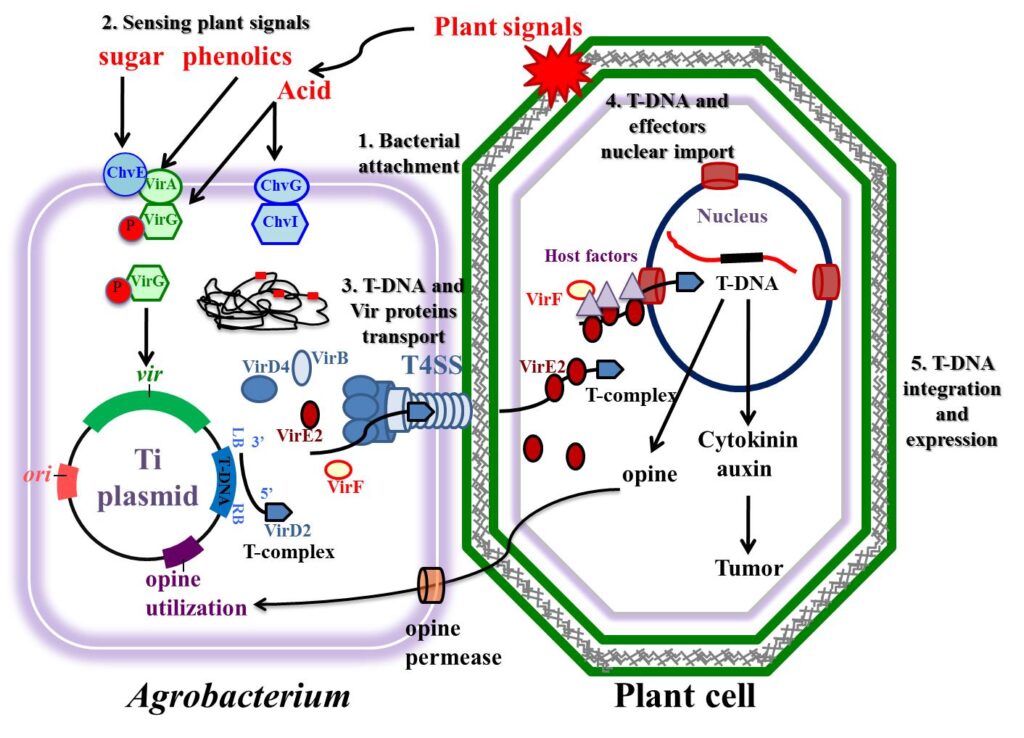
Agrobacterium tumefaceins is a bacterium responsible for causing the crown gall disease in a variety of dicotyledonous plants. The mechanism of causing crown gall disease is by insertion of small segment of DNA called as transfer DNA (T-DNA) from Agrobacterium tumefaceins to plant cells hence, scientist thought of exploring it for transferring desired genes.
Agrobacterium system was historically the first successful plant transformation in plant genetic engineering in 1983. It provides the natural gene transfer, gene expression and selection systems. Large plasmids in these agrobacteria are called tumor inducing plasmid (Ti-plasmid) and root inducing plasmid (Ri-plasmid) which are responsible for causing the crown gall diseases in dicot plants. It was observed that not whole plasmid but only a small part of the Ti-plasmid called the T-DNA (transfer DNA) is transferred to and integrated into the host plant nuclear genome. The size of T-DNA is approximately 23Kbp and is responsible for the cancerous properties of the transformed cells. It also synthesize opines. In the Ti-plasmid, T-DNA is flanked by 25bp direct repeats on both the sides. These sequences play major role in the integration of T-DNA into the plant genome. Agrobacterium has proved to be an icredible useful tool for the integration of genes into plants.
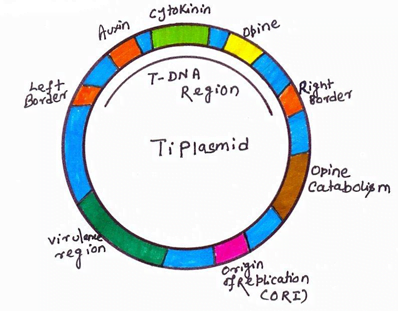
Major steps of the Agrobacterium tumefaciens-mediated plant transformation process.
(1) Attachment of A. tumefaciens to the plant cells.
(2) Sensing of plant signals by A. tumefaciens and regulation of virulence genes in bacteria following transduction of the sensed signals.
(3) Generation and transport of T-DNA and virulence proteins from the bacterial cells into plant cells.
(4) Nuclear import of T-DNA and effector proteins in the plant cells.
(5) T-DNA integration and expression in the plant genome.
Advantages –
- It is a natural means of transfer and it is therefore perceived as a more acceptable technique to those who feel natural is the best.
- Agrobacterium is capable of infecting intact plant cells, tissues and organs and as a result tissue culture limitations are much less of a problem.
- Agrobacterium is capable of transferring large fragments of DNA very efficiently without substantial rearrangements.
- Integration of T-DNA is a relatively precise process.
- Stability of transferred gene is excellent.
Disadvantages –
- It has limitation of host range, some important crops cannot be infected with agrobacterium.
- Sometimes cells in a tissue that are able to regenerate are difficult to transform.
Microprojectile Bombardment–
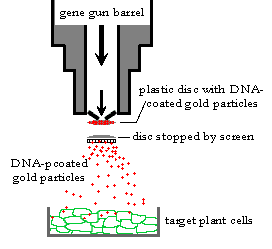
The technique of particle bombardment also known as biolistics, microprojectile bombardment, particle acceleration etc, is the most versatile and effective way for the creation of many transgenic organisms. The procedure in which high velocity microprojectile were utilized to deliver nucleic acids into living cells was described by KLEIN and SANFORD in 1987.
The desired gene is coated on tungsten or gold particles (3 micrometer in diameter) called as microprojectiles. These are accelerated with pressure on the plant cells or tissues. The microprojectile is stopped by perforated plate while allowing microprojectile to pass through it. When microprojectiles enters the plant cells, the desired genes are released from microprojectiles and may get inserted into the plant chromosomal DNA and get transformed. DNA markers are used to distinguished the transformed and non-transformed cells. The transformed plant cells with desired characteristics are further developed to whole plants.
Advantages –
- It is clean and safe
- Transformation of recalcitrant species is possible with this method
- Transformation of organized tissue
- It is possible to study the basic plant development process.
Disadvantages –
- In plants gene transfer leads to non-homologous integration into the chromosome, and is characterized by the multiple copies and some degree of rearrangement.
- The emergence of chimera plants
- Lack of control over the velocity of bombardment which leads to substantial damage to the target cells.
Electroporation-
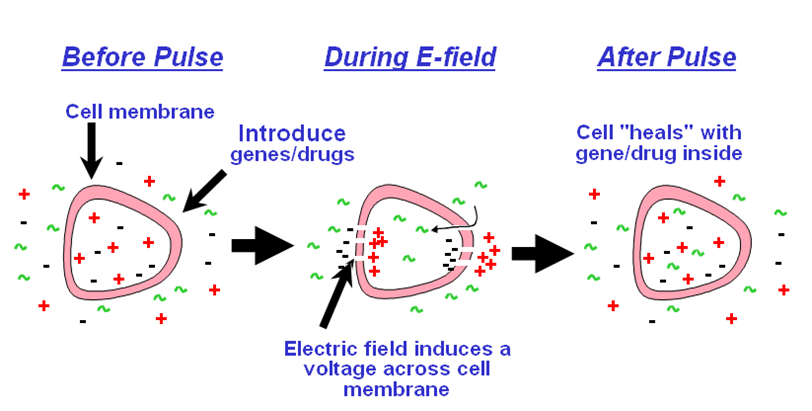
Electroporation is the process where the electrical impulses of high field strength are used to reversibly permeabilize cell membranes to facilitate uptake of large molecules, including DNA. In this procedure, a sample of protoplasts is pulsed with high/low voltage pulses in the chamber of an electroporator. The electric pulse disrupts the phospholipid membrane and form temporary pores making it permeable. The electric pulse also cause change in electric potential across the membrane allowing the charged molecule namely DNA or RNA to move across the membrane. It uses relatively high initial field strength (1-1.5Kv) with a low capacitance and therefore a short decay time. It has been reported that the linear DNA is transfected efficiently than circular DNA.
https://www.btxonline.com
Gene Transfer By Polyethylene Glycol-
This technology is applicable for protoplast only. Protoplast means cells without cell wall. polyethylene glycol is polyether used and it is used to stimulate endocytosis and thereby causing the uptake of DNA. PEG causes precipitation of DNA and stimulate endocytosis. In the process, the liner DNA with desired gene is incubated with protoplasts in presence of Mg+ and Ca2+. The concentration of PEG used is 15% having 8000 dalton molecular weight.
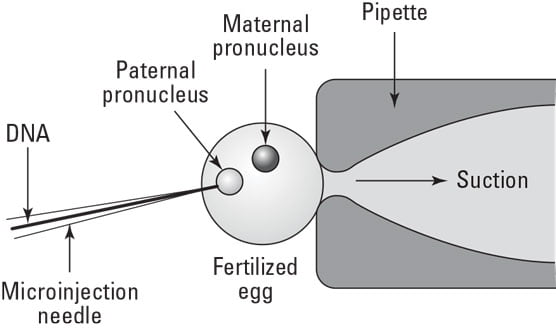
Microinjection –
It is the direct mechanical method for gene transfer in target. A target can be a defined cell within a multicellular structure such as embryo, ovule, and meristem or protoplasts, cells or a defined compartment of a single cell. Microinjection is able to penetrate intact cell walls. Glass micropipettes with 0.5-10.0 micrometer diameter tips are used to transfer the micromolecules into the cytoplasm or the nucleus of a recipient cell or protoplast. Once injection has been achieved the injected cells must be cultured properly to ensure its continued growth and development.
Liposome Mediated Gene Transfer-
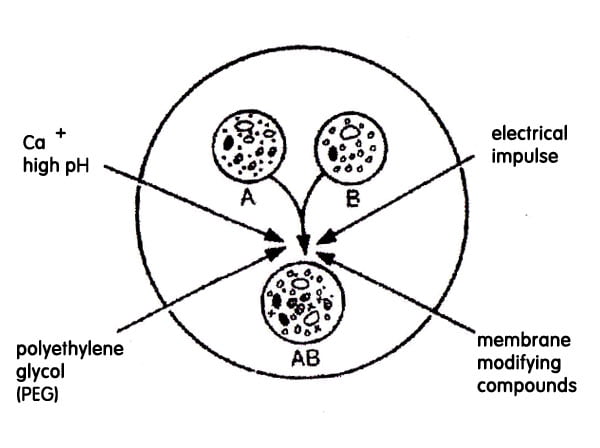
Liposomes are artificial lipid vesicles surrounded by a synthetic membrane of phospholipids. They are amphipathic in nature consisting of hydrophilic and hydrophobic molecules. They are fluidic in nature. These can be induced to fuse the protoplasts using PEG and have therefore been used for gene transfer. In this method, DNA enters the protoplasts due to endocytosis of liposomes aand it involves the below steps:
- Adhesion of liposomes to the protoplast surface.
- Fusion of liposomes at the site of adhesion.
- Release of plasmids inside the cells.

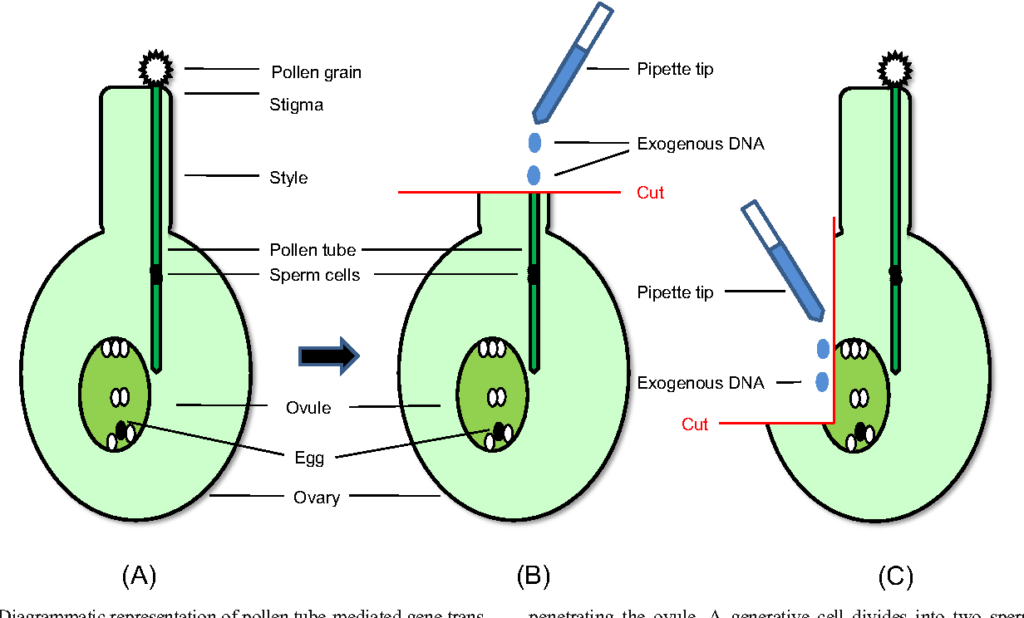
Gene Transfer Via Pollen Tube Pathway-
Pollen has been suggested as a vector for gene transfer by various worker. It has been reported that introduction of DNA into gametes followed by fertilization and zygotic embryogenesis will result in gene transfer. In this method after pollination the styles are been cut and DNA is inserted through it. The DNA reaches the ovule by flowing down the pollen tube. This procedure is called pollen tube pathway of gene transfer. It was first applied for the transformation of rice.
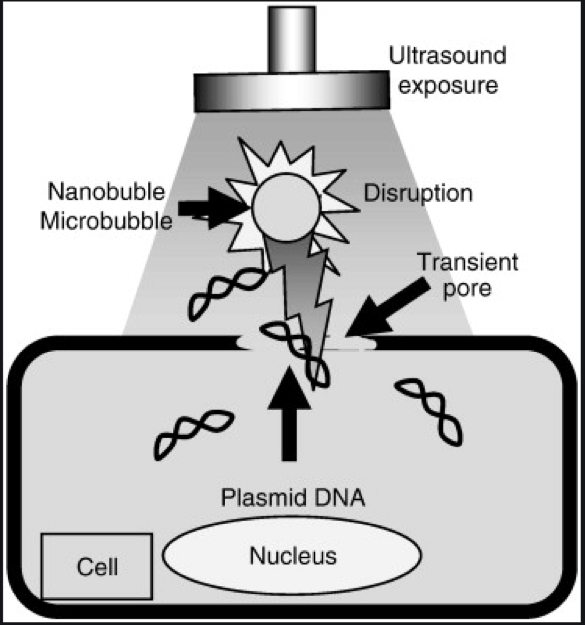
Ultrasound method DNA transformation-
Ultrasound is used for stimulating uptake of foreign DNA by plant protoplast and leaf segments of tobacco. The procedure involves immersion of explants in sonication buffer containing plasmid DNA and sonication with an ultrasonic pulse generator at 0.5w/cm acoustic intensity for 30 min. Samples are rinsed in a buffer solution and then cultured for growth and differentiation. This technique has the advantages of being simple, inexpensive and multifunctional. One can use standardized conditions and there is no requirement for tissue culture expertise. Little success has been achieved by this technique.
References:
Zaenen, I., N. Van Larebeke, M. Van Montagu, J. Schell. 1974. Supercoiled circular DNA in crawn gall inducing Agro bacterium strains. J. Mol. Biol., 86 (1): 109-127.
Van Larebeke, N., G. Engler, M. Holsters, S. Van de n Elsacker, L. Zaenen, R.A. Schilperoort, J. Schell. 1974. Large plasmid in Agrobacterium tumefaciens es essential for crown gall inducing ability. Nature
Yadav, N.S., J. Vanderleyden, D.R. Bennett, W.M. Barnes, M.D. Chilton. 1982. Short direct repeats flank the T-DNA on a nopaline Ti plasmid.
Pua, E.C., A. Mehra-Palta, F. Nagy, N.H. Chua. 1987. Transgenic plants of Brassica napus L. Biotechnology.
Hiei, Y., S. Ohta, T. Komari, T. Kumashiro. 1994. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium. and sequence analysis of the boundaries of the T-DNA.
Schlappi, M., B. Holm. 1992. Competence of immature maize embryos for Agrobacterium-mediated gene transfer.
Sanford, J.C. 1988. The Biolisfic Process. Trends Biotech.
Fisk, H.J., Dandekar, A.M. 2005. Electroporation: introduction and expression of transgenes in plant protoplasts. Methods Mol Biol.
Miao Y, L. Jiang. 2007. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat Protoc.
Jogdand, S.N. (2006). Gene Biotechnology. Himalaya Publishing House. Mumbai, India.
Crossway, A., J.V. Oakes, J.M. Irvine., B. Ward, V.C. Knauf, C.K. Shewmaker. 1986. Integration of foreign DNA following microinjection of tobacco mesophyll protoplasts. Molecular and General Genetics.
Song, X., Y. Gu, G. Qin. 2007. Application of a transformation method via the pollen-tube pathway in agriculture molecular breeding. Life Science Journal.
Luo, Z., Ray W. 1988. A simple method for the transformation of rice via the pollen-tube pathway. Plant Mol Bio Reporter.
Trick, H.N., J.J. Finer. 1997. SAAT: sonication assisted Agro bacterium -mediated transformation. Transgenic Res.
Christou, P. 1996. Transformation technology. Trends Plant Sci.