A pH indicator is basically a chemical compound which is added to a solution to visually determine the pH (acidity or basicity) of the solution. The characteristic color changes indicate the acidic, basic, or neutral character of the substance being tested.
Introduction to pH Indicator
pH indicators or acid-base indicators are chemical substances, which indicate the change in pH. These indicators are used in Microbiology to identify the change in pH caused by microbial metabolic activity. The change in the pH is indicated by change in their color and hence, visually we can determine it. Chemically, these indicators are weak acid or bases. The weak acid or bases are those, which ionize, partially in aqueous medium (Strong acid/base ionize completely). The pH indicators are also called as natural dyes, which indicate the concentration of hydrogen or hydronium ions. The pH papers are the paper strips that are soaked in such dyes.
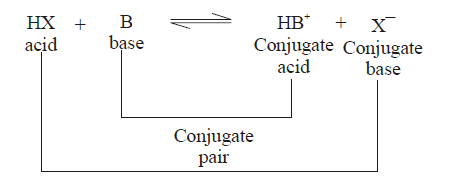
The ability to indicate change in color is because the weak acid and its conjugate base have different color. For example, the very well known pH indicator phenolphthalein (used in chemistry and Microbiology) is colorless at acidic and neutral pH, but becomes pink in color in alkaline media because the weak base conjugate of phenolphthalein is pink in color.
A wide range of pH indicators are used in Microbiology. This need for wide range of pH indicators is because of their difference of bringing change in color is varied at different pH. The pH indicator is selected depending upon the aim and the requirement of the experiment.
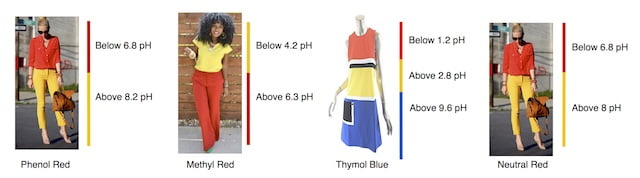
Following table enlists few pH indicators used in Microbial media –
| pH Indicator | Mechanism | Exist in color | pH range |
| Phenol Red | The phenol red has negative sulfate group and positive ketone group hence it is present in zwitter form (with both positive and negative charge). At acidic pH, the ketone group is lost and the color is changed from red to yellow. | Red | 6.8 to 8.2 |
| Methyl red | The methyl red has carboxyl group and at alkaline pH, it loses its proton and become yellow in color | Red | 4.4-6.2 |
| Thymol Blue | The thymol blue has two proton, it donated one proton around 1.2 pH causing change in color from red to yellow and other proton is lost around 8 pH causing blue color formation. | Brownish Green or Reddish Brown in color | 1.2 to 2.8 and 8.0 to 9.6 |
| Neutral Red | The Neutral red donate the proton from amino group at 6.8 pH and causes change in color from red to yellow | Red color | 6.8 – 8.0 |
Here, we are sharing some images that would help you to remember the difference between pH indicators.
BONUS
Activity:
Take a picture wearing such color combination and share it on social media platforms. Tag us and mention which pH indictor are you representing.
#pH indicators # Microbiology

Need more help? Click here.
If you liked this resource, please Like, Share, and Subscribe us for more content.