Calculations is one of the major challenge before Life Science Students.
Introduction:
As a Science graduate, we all know the importance of basic calculations in our Academic life. If we do not have good command over the basic calculation, it is difficult to excel in our laboratory work. The laboratory allows us to explore and test the theoretical concept that we had learned in theory classes. The basic requirement of any Science laboratory is to prepare various solutions of different concentration and hence we must know what is the solution & what is the concentration?
Solution is defined as the homogenous mixture of two or more component namely solute and solvent. Whereas, the quantity of solute present in the definite solution relative to the solvent is known as concentration of solution or strength of solution. In this article we will learn all the basic concepts and calculation that we need in our laboratory work.
Type of solutions :
In laboratory, we make majorly two main types of solutions:
- Standard solutions: The solution, which has known concentration.
- Dilute solutions: The solution, which contains low quantity of solute relative to the solvent
Units used to express concentration:
There are different ways of expressing the concentration of solution and they are as follows-
- Weight fraction or weight (W/W) %
- Volume fraction or volume (V/V)%
- Weight volume fraction (W/V) %
- Molarity (M)
- Normality (N)
- Molality (m)
- Mole fraction (X)
Calculations –
Let’s understand them one by one with sample problems –
- Weight fraction (w/w)% : Weight fraction is termed as how much gram solute is dissolve in 100 grams of solution.
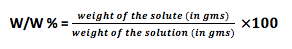
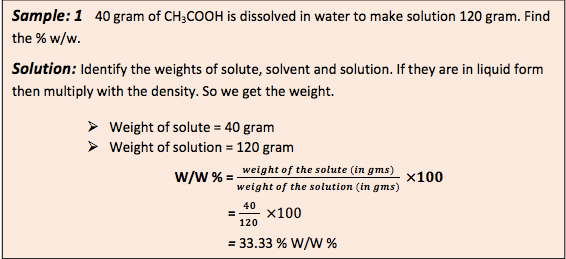
Let’s use the formula and try to solve some calculations-
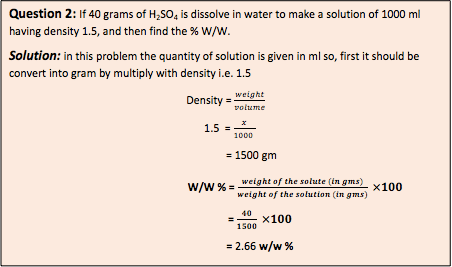
- Volume fraction (V/V)% : Volume fraction (V/V) % is defined as number of millimeters of solute dissolved in 100 ml of the solution.
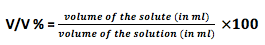
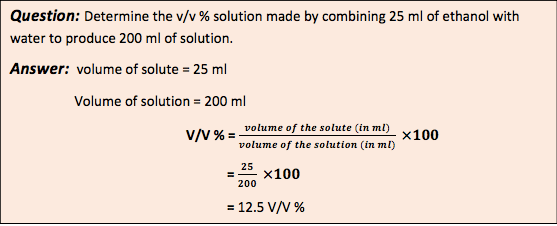
- Weight volume fraction (w/v) % : The how much gram of solute dissolve in 100 ml solution is define as weight fraction.
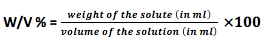

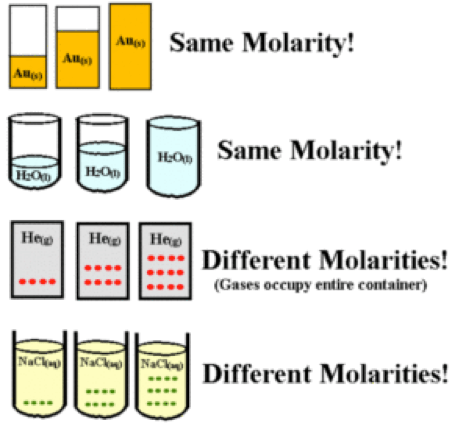
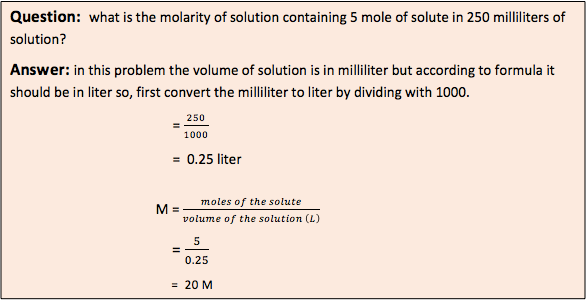
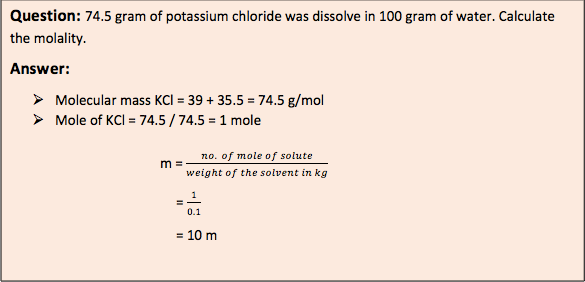
- Molarity (M): It is defined as mole of solute per liter of solution at given temperature (moles / liter). As the temperature is increased, the molarity gets decreased due to decrease in solvent volume.
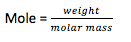
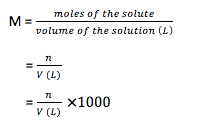
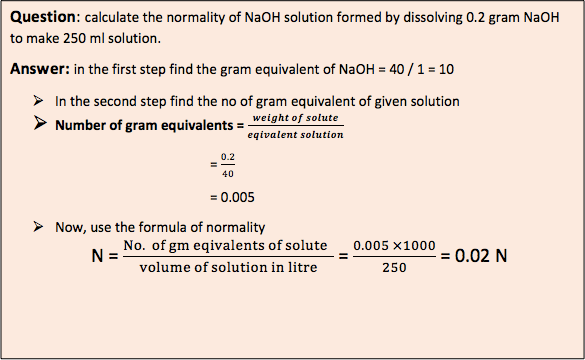
- Normality (N): It is defined as number of gram equivalents of solute per liter of solution (gram equivalents /liter).
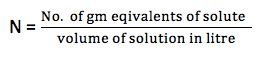
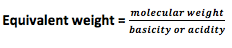

- Molality (m) : It is defined as mole of solute present in 1000 gram of solvent (moles/kg). As the solvent is in solid state, temperature does not affect the Molality.
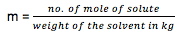
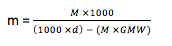
The above formula explains the relationship between Molarity and Molality
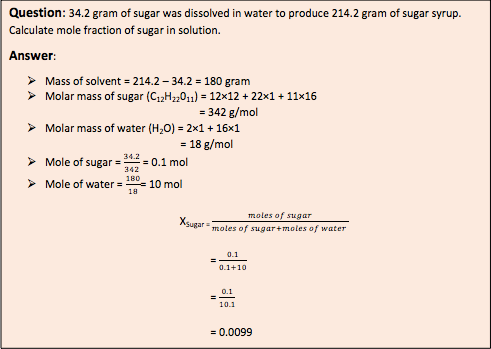
Mole fraction (X) : It is the ratio between numbers of mole of one particular component to the total number of moles of all the components of solution. It has no units. It is independent of temperature. The sum of mole fraction of all component is always 1. i.e x1 + x2 = 1.
Relation between molality and mole fraction.
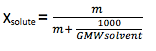
References
https://www.ausetute.com.au/wtvol.html
https://www.pharmaguideline.com/2018/07/preparation-of-molar-and-normal-solutions.html
https://www.toppr.com/guides/chemistry/solutions/normality-formula-definition-and-examples/

Good job bro keep it up 😊❤️
Thank you so much