Nucleotide and Nucleoside are the building blocks of Nucleic acid. To understand the structure and function of Nucleic acid, it is important to learn the fundamentals of Nucleotide and Nucleoside.
Introduction to Nucleotide and Nucleoside:
DNA is a polymer of nucleotide. It consists of two anti-parallel polynucleotide strands that intertwined in the right-handed direction. The two strands are hold together by the hydrogen bonds. DNA is called as a hereditary material because it transfers the crucial information from one generation to another. The information of protein synthesis is stored in the form of sequence of nucleotides.
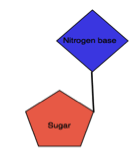
Nucleoside: It is an organic molecule consisting of nitrogen base (Purine or Pyrimidine and 5-C sugar.
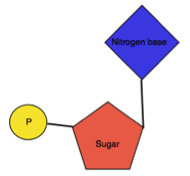
Nucleotide: It is an organic molecule made from nucleoside and phosphate.
Difference between Nucleotide and Nucleoside
| Nucleoside | Nucleotide |
| It made up of Sugar and Nitrogen base | It is made up of Sugar, Nitrogen base and Phosphate |
 |  |
| It is slightly alkaline | It is slightly acidic because of phosphate group |
| It is precursor of Nucleotide | It is precursor of DNA |
Nitrogen Bases of Nucleotide and Nucleoside:
Nitrogen bases are the nitrogen containing organic cyclic molecule. They are called as bases because of its ability to accept the hydrogen ion. Similar to Acid-base concept, the nitrogen bases also reduces the concentration of hydrogen ions hence called as bases.
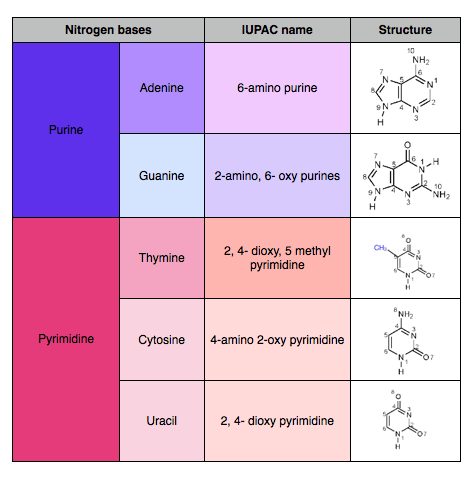
The Nitrogen bases are of two types namely Purines and Pyrimidines. Adenine and Guanine are Purines while Thymine and Cytosine are Pyrimidines. Purines are 9 member containing 2 closed rings and Pyrimidines are 6 member containing single closed ring.
Common characteristics of purines and pyrimidines are:
- They both are hetero-cyclic.
- They are aromatic in natures.
- They absorb UV at 260nm.
- They can form hydrogen bonds.
Adenine, guanine, thymine and cytosine bases are found in DNA whereas in RNA, Uracil is present in place of thymine.
Sugar of Nucleotide and Nucleoside:
The sugar present in nucleotide is a 5 member ribose type. It has an aldehyde as a functional group. Its cyclic structure is called as furanose because its ring contains 4 carbons and 1 oxygen. To differentiate the numbering of atoms of nitrogen base and sugar in nucleotide and nucleoside structure, the sugar atoms are numbered with (‘), and it is read as prime. In RNA, the pentose sugar is ribose whereas in DNA, the pentose sugar is 2’-deoxyribose. 2’-deoxy means that oxygen is absent at 2’ C of ribose sugar.
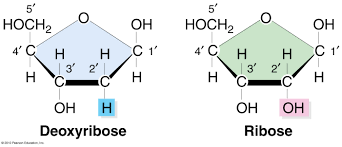
The furanose ring is in wrinkled form and hence, it is a non planar. The non-planarity structure of sugar is called as sugar puckering. In the given image, the different carbon position is pictured. In DNA, C2’endo and C3’endo is found.
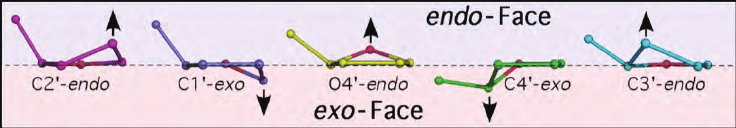
A distinct function of sugar puckering has been observed in DNA replication and Transcription. In DNA replication the DNA polymerase bind DNA to C2’endo conformation. In transcription the RNA polymerase bind DNA to C3’ endo conformation. The sugar puckering also plays a vital role in forming different forms of DNA (A, B and Z).
Alpha and Beta isomer in Nucleotide and Nucleoside sugar-
Ribose and deoxyribose sugar have two isomers i.e. alpha and beta. In an alpha ribose or deoxyribose sugar, the hydroxyl group at C1 (anomeric carbon) is in downward orientation. In beta, the hydroxyl group at C1 is in upward direction. (Tips to remember the difference – Beta is upstairs).
In DNA and RNA, the furanose sugar is of beta isomer form. The beta isomer allows formation of glycosidic bond with nitrogen base.
Furanose sugar and Nitrogen bases are linked by glycosidic bond. It is a covalent bond.The C1’ of furanose sugar is linked to nitrogen base pair at N9 of pyrimidine and N1 of Purine. When any of the purine or pyrimidine is linked to furanose sugar, it is called as Nucleosides.
Minor bases:
Adenine, Guanine, Thymine and Cytosine are called as major base. Minor base pairs are the modified form of the major base pairs. They are called as minor because they are present in very less number as compared to the major bases. The modified base pair may have additional groups like methyl, hydroxymethyl and glucosyl etc. The modified bases may play major role in regulating gene expression and DNA supercoiling. The structure and function of some minor bases are as follows-
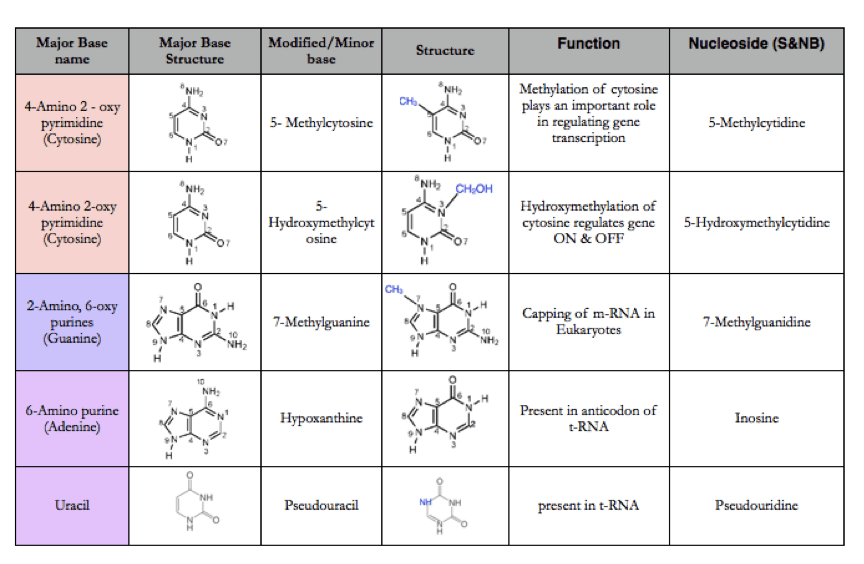
The nomenclature of Nucleosides and Nucleotides is as follows-
| Nitrogen Base | Nucleoside | Nucleotide |
| Adenine | Adenosine | Adenylate |
| Guanine | Guanosine | Guanylate |
| Thymine | Thymidine | Thymidylate |
| Cytosine | Cytodine | Cytidylate |
Nucleotide
When Nucleosides are linked to phosphate group at C5’ of furanose sugar, it is called as Nucleotides. The phosphate group is linked to sugar by phosphoester bond. One, two or three phosphate groups may be present in nucleotides. The nomenclature is based on the number of phosphate group present and it is as follows-
| Nitrogen Base | One Phosphate | Two Phosphate | Three Phosphate |
| Adenine | Adenine 2’ –monophosphate (AMP) | Adenine 2’ –diphosphate (ADP) | Adenine 2’ –triphosphate (ATP) |
| Guanine | Guanine 2’ –monophosphate (GMP) | Guanine 2’ –diphosphate (GDP) | Guanine 2’ –triphosphate (GTP) |
| Thymine | Thymine 2’- monophosphate (TMP) | Thymine 2’-diphosphate (TDP) | Thymine 2’-triphosphate (TTP) |
| Cytosine | Cytosine 2’ monophosphate (CMP) | Cytosine 2’ diphosphate (CDP) | Cytosine 2’-triphosphate (CTP) |
| Structure | 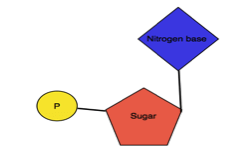 | 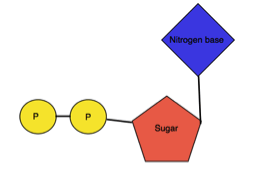 | 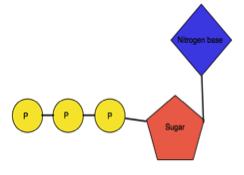 |
Function of Nucleotides:
- ATP is the universal currency of energy to run the metabolic and molecular pathways of living cells.
- Cyclic form of AMP acts like messenger in the hormonal pathways of eukaryotic organisms.
- ATP activates and inhibits many of the important enzymes of metabolic pathways.
- Few nucleotides act as the important component of co-enzymes.
- Adenine nucleotide is a vital constituent in forming NAD (P)+, FAD, and CoA structure and function.
References:
http://med.fau.edu/students/md_m1_orientation/Chapter9.pdf
https://library.med.utah.edu/NetBiochem/pupyr/pp.htm
https://uh.edu/dtu/19-Nuc%20Mata-1-08.htm
http://www.bioinfo.org.cn/book/biochemistry/chapt12/bio7.htm
Lehninger, Nelson, D., and Cox, M.; W.H. Freeman and Company, New York, 2005, 1216 pp., ISBN 0‐7167‐4339‐6
Biochemistry, 5th edition, Jeremy M Berg, John L Tymoczko, and Lubert Stryer, New York: W H Freeman; 2002. ISBN-10: 0-7167-3051-0



One thought on “Fundamentals of Nucleotide and Nucleoside”