Gas Chromatography is an analytical technique used for separation of components or chemical species from the mixture.
Introduction to Gas Chromatography –
Chromatography is defined as an analytical technique for isolation, identification and quantification of biological sample. It is one of the major techniques employed in research and Industries for analytical studies. All the chromatographic technique consist of two immiscible phases – Stationary and Mobile phase. The basic principle of Chromatography technique is different components of mixture or samples have different affinity towards two immiscible phases. This differential interaction with stationary and mobile phase allows the separation of individual components from mixture. The sensitive detectors identify the separated components.
There are different types of chromatographic techniques based on types of bed, mechanism of separation and physical state of two immiscible phases. Read our article on different types of chromatography.
Gas Chromatography is an analytical technique where the physical state of stationary phase is liquid and mobile phase is gas, hence called as Gas chromatography. In Gas Chromatography, the stationary phase is adsorbed on inert solid material that is either packed or immobilized on the surface of column. It is most suitable of volatile sample.
Components of Gas Chromatography –
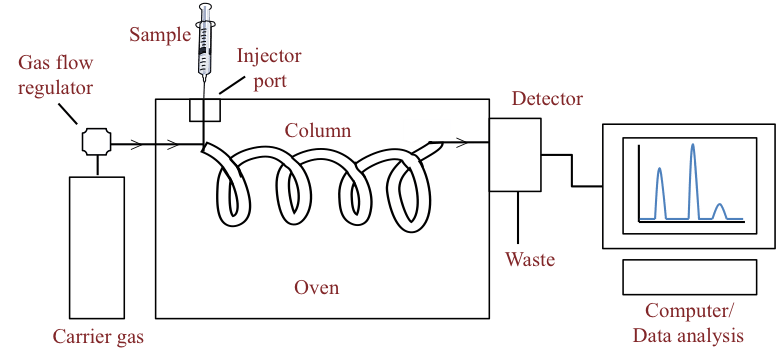
Carrier gas –
- It is the mobile phase that runs in column.
- Hydrogen, helium, argon or nitrogen is mostly used as carrier gas.
- The helium gas is mostly preferred because of its efficiency and safety.
- The carrier gas is filled in reservoir tank and regulator controls the flow of gas.
- The carrier gas must be pure.
- It is equipped with molecular sieve for filtering and removal of moisture and other impurities if present.
Sample injector –
- The sample that needs to be analyzed is injected in Gas chromatography through sample injector.
- To inject the sample, calibrated syringe is used.
- As the sample needs to travel with mobile phase they both should be in same physical state. In Gas Chromatography, the mobile phase is in gaseous state hence sample also need to be gaseous state.
- The sample injector is equipped with heater that allows the vaporization of liquid sample.
- If sample is in solid state, it is crushed and grinded and converted to liquid state.
Column –
- The column consists of stationary phase (liquid adsorbed on inert solid support).
- The column is mostly made from stainless steel.
- The column can be packed with stationary phase called as packed column or thin layer of stationary phase is bonded on the inner walls of column forming hollow tube called open tubular column
- The length of column used in gas chromatography is in range from 1.5 to 10m with diameter of 2 -4 mm. It is present in the form of coil.
- The column is present inside the oven. The temperature of oven is controlled and monitored by the software.
- The temperature of oven increases gradually.
- The high temperature of oven ensures the sample to stay in gaseous state.
- The optimum temperature of oven is depends on sample’s boiling point and hence it is varies from sample to sample.
- When the sample is injected via injector, it travels with carrier gas (mobile phase) in column filled with stationary phase. The components of mixture (sample) interact with both immiscible phases in differently.
- Due to difference in the interaction with stationary and mobile phase, the time taken by individual components to travel the column is different.
- The time spend by individual component in the column is called as retention time. This characteristic is used for identification of sample
Detector –
- The Detector measures the quantity of the components or ingredients of sample.
- There are different types of detectors available. The working mechanism of different detectors varies. They also differ in selectivity i.e. ability to quantify based on molecule’s physical and chemical property.
- The most commonly used detectors are Flame Ionization and Thermal conductivity detector.
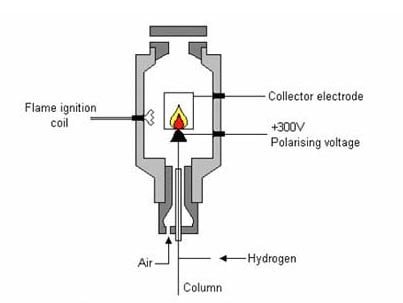
Flame Ionization detector – When the carrier gas that carries the sample in column and enters the detectors and passes through hydrogen air flame. It causes chemical decomposition and ionization of sample. The collector collects the produced ions. It causes increase in the current. The current is directly proportional to quantity of sample that is burned. Hence, the number of ions (produced during ionization) depicts the concentration or quantity of sample. The produced current is converted into digital form and present in the output device. As the works on ionization caused by burning of sample in hydrogen flame, the detector is called as Flame Ionization detector.
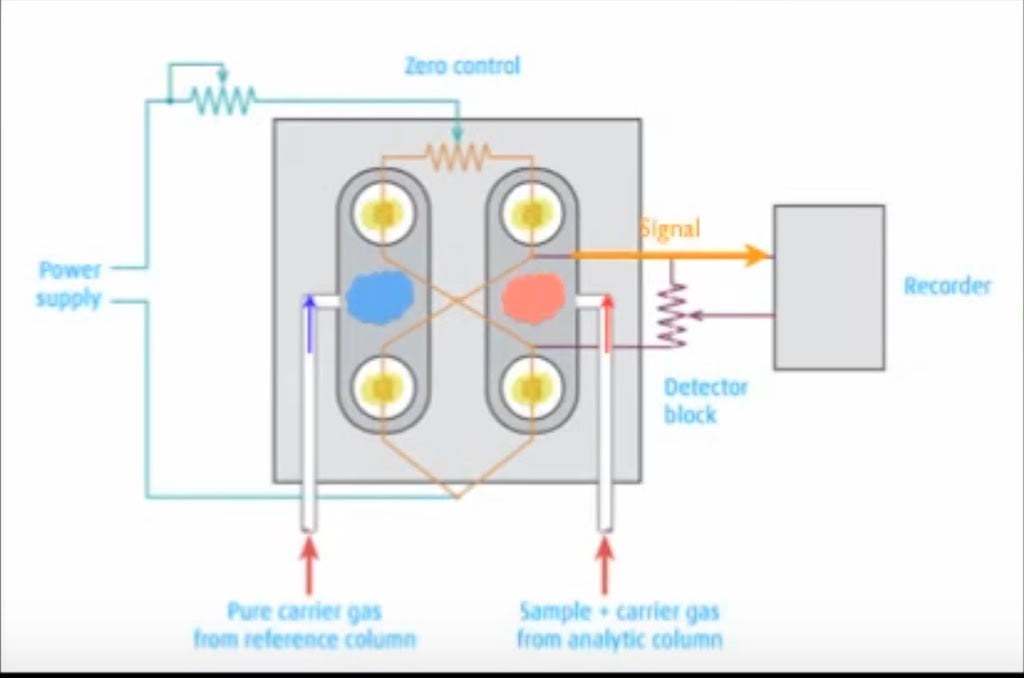
Thermal conductivity Detector – This detector works by measuring the difference in thermal conductivity caused by flow on carrier gas with sample with reference to flow of only carrier gas. TCD is also located at the end of the column. It consists of paired tube like thermistors. When carrier gas and sample travels through tube, it causes change in the temperature. This change in temperature is sensed by Wheatstone bridge.When pure carrier gas is passed through both the tubes, there is no difference in the temperature of the tubes and hence bridge is balanced. When one tube carries pure carrier gas while carries carrier gas with sample, the bridge become unbalanced. It is because of difference in temperature of both the tubes. The extent of difference of temperature is directly proportional to the concentration of sample. The difference in the temperature is measured and recorded and converted to digital signal and presented in output device.
Applicationsof Gas Chromatography –
Gas Chromatography is exclusively used for isolation, identification and quantification of volatile compounds or mixtures. Such analytical studies need to be conducted in Pharmaceutical and cosmetic industries, Medical fields and Environmental studies.
References–
http://hiq.linde-gas.com/en/analytical_methods/thermal_conductivity_detector.html
https://www.analyzertechs.com/tcd.html
https://teaching.shu.ac.uk/hwb/chemistry/tutorials/chrom/gaschrm.htm
http://hiq.linde-gas.com/en/analytical_methods/
Dr. Sangha Bijekar has 9 years of Teaching Experience at University level. She loves to get engage in teaching and learning process. She is into blogging from last two years. She intends to provide student friendly reading material. She is avid Dog Lover and animal rescuer. She is learned Bharatnatyam and Katthak Dancer. She is into biking and She also loves to cook.


