Chromatography is an analytical technique used for isolation, separation and identification of biomolecule.
As a life Science student or researcher, we need to work on different biomolecules. We may need to separate the biomolecules from the mixture or may be need to identify them. For identification and separation of biomolecules, analytical techniques have been invented. Chromatography is one of such analytic methods. It is defined as an analytical technique used to separate the components from mixture and analyze the individual component.
Chromatography method was developed in 1903 by Mikhail Tswett. He used this method to separate the mixture of plants’ pigments. He exploited the natural capillary phenomena of paper and cloth for the separation of pigments. He dissolved the plant pigments in water or alcohol. He brought paper or cloth into the contact of dissolved pigments. Due to the capillary action, the pigments traveled but not in the same speed. lighter the pigment, faster it was traveling along the cloth or paper. Due to the difference in the weight of the pigments, the pigments formed different bands of color. Individual band represents the individual pigment. As a result of formation of colored bands, Mikhail Tswett named this technique as Chromatography. ‘Chroma’ means color and ‘Graphy’ means writing. In the honor of his contribution of Mikhail Tswett is called as Father of Chromatography.
Principle of Chromatography:
The Chromatography technique works on facts that the sample distribute, separate or interact differently to two immiscible phases (A and B). The extent of interaction with two immiscible phases depends on the chemistry of sample. The formula that derives the relation between the sample and two immiscible phases is
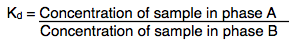
Kd is called as partition or distribution coefficient. It is an equilibrium constant that represents the ratio of concentration of sample that has been distributed in two immiscible solvents. The Kd values indicate the solubility biomolecules in the selected solvents. Kd value is intensely used in Pharmaceutical Industry.
The Two immiscible phases can be any of the physical state – solid, liquid or gas. The one phase is called as Stationary phase and other is Mobile phase. The stationary phase can be solid or liquid supported in solid surface. The mobile phase can be liquid and gas state.
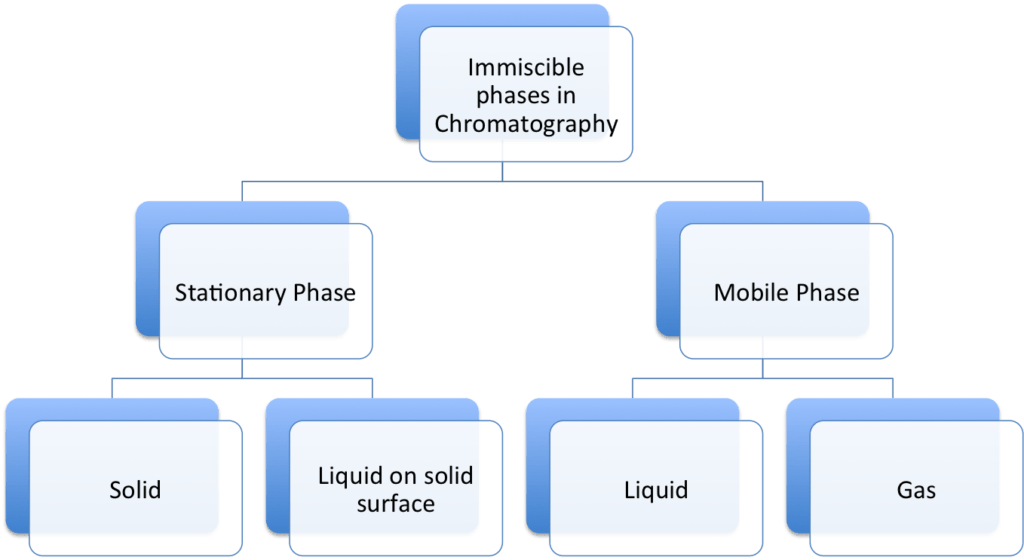
Types of Chromatography:
As the research has advanced different types of chromatographic techniques have been developed based on bed design, mechanism of separation, physical state of stationary and mobile phase.
Based on design of bed –
The Chromatography types are planar and column. In planar Chromatography, the stationary phase is present as or on a plane. It can be paper or any other stationary phase like silica or alumina gel soaked or impregnated on solid support (mostly glass or plastic). In column chromatography, the solid stationary phase is filled in a tube or a column. If the stationary phase is in liquid state then it is coated on solid surface. If the column is completely filled with stationary phase then packed column. If the stationary phase is coated only the surface of the walls of column leaving hollow space at the center of column then it is called as open tubular column.
Based on physical state of immiscible phases –
The Chromatography are of three types based on the physical states of two immiscible phases namely Liquid Chromatography (Solid-liquid and liquid-liquid) and Gas Chromatography (Gas-liquid and Gas-solid).
In Solid-Liquid Chromatography, where the stationary phase is solid and mobile is in liquid state. In Liquid-Liquid Chromatography, both the immiscible phases are in liquid state. One of the stationary liquid is supported using solid surface.
In Gas-solid Chromatography, the stationary phase is of solid and mobile phase is gas whereas in Gas-liquid Chromatography, the stationary phase is liquid and mobile phase is gas.
Based on the mechanism of separation:
- Partition Chromatography: It works on the principle that the individual components of mixture have different affinity towards the two immiscible phases. The difference in the affinity is due to the difference in the physical and chemical properties of individual chemical component of the mixture. Based on the affinity, the components of the mixture forms non covalent bond with two immiscible phases gets distributed or separated.
- Adsorption Chromatography: It works on the principle that components of mixture have different affinity towards an adsorbent. An adsorbent is a material which adsorbs any substance because of non covalent interaction. Out of the two phases, one phase is an adsorbent example silica gel, alumina gel etc.
- Size exclusion Chromatography: It separates the components of the mixture on the basis of size and shape. It consist of porous gel like material as a stationary phase and liquid as a mobile phase. The pores of porous gel are available in different sizes. The size of the pores are of similar size to biomolecule’s size. when sample is loaded in column filled with porous gel, the smaller components of the mixture penetrates into pores while the larger molecule remain outside. The larger molecule elute or exit first and allow us to separate the mixture of biomolecules of different sizes.
- Affinity Chromatography: The Affinity Chromatography exploits the specific relationship of enzyme and substrate. Its column consist of support medium on which the substrate are immobilized. When mixture of enzymes or proteins are flooded in the column, the proteins and enzymes that have binding sites for immobilized substrate with bind to it. Whereas the non binding proteins and enzymes will leave the column. Hence, the components of mixture are separated based on the affinity for substrate.
- Ion Exchange Chromatography: Ion Exchange Chromatography uses positive and negative ion interaction. This Chromatography is specifically used for separating charged molecules. The column consist of either of ions (positive or negative) immobilized on support media. When mixture of ions is poured in column, the immobilized ion selectively binds the opposite charged ion and separates it from the mixture.
Applications of Chromatography:
- It is used for separating the individual components from mixture.
- It is used for identifying the unknown biomolecule.
- It is used for quantifying the biomolecule.
- It is used for characterization of biomolecules.
MCQ test –
Solve the Test and check your score here.
References:
http://www.umich.edu/~orgolab/Chroma/chromahis.html
https://www.soinc.org/sites/default/files/uploaded_files/forensics/For_Chromatography3.pdf
http://www.chemguideforcie.co.uk/section112/learningf.html
https://www.ucl.ac.uk/~ucbcdab/enzpur/affinity.htm
Dr. Sangha Bijekar has 9 years of Teaching Experience at University level. She loves to get engage in teaching and learning process. She is into blogging from last two years. She intends to provide student friendly reading material. She is avid Dog Lover and animal rescuer. She is learned Bharatnatyam and Katthak Dancer. She is into biking and She also loves to cook.


One thought on “Principle, Types and Applications of Chromatography”