pH meter is used to measure acidity or alkalinity of solutions. pH meter is an electronic instrument It measures the activity of Hydrogen ions. It is widely used in various branches of Science.

Introduction:
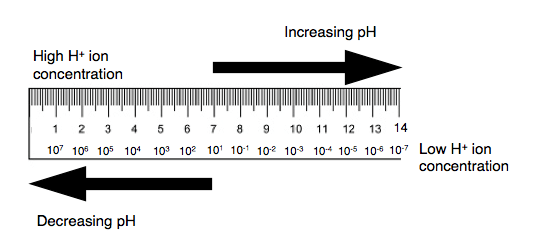
pH indicates the Hydrogen ion concentration of any given solution. pH signifies the power of hydrogen. To understand the theory of pH, it is important to know about dissociation of water. The pH scale has been derived from spontaneous dissociation of water. The water spontaneously dissociate into its H+ and OH- components. The concentration of H+ ion found in pure water is 1 x 10-7. This H+ ion concentration is neither acidic nor alkaline and hence called as neutral.
When the H+ ion concentration is more than 1 x 10-7, the solution is acidic; when H+ ion is less than 1 x 10-7, then the solution is alkaline. Low pH indicates high H+ ion concentration; High pH indicates low H+ concentration. Hence the pH scale inversely related to concentration of H+ in any given solution; and directly related to OH– ion concentration.
pH = -Log10 [H+]
The pH scale is in the range from 1 to 14. When there is change of pH by unit 1 (for ex. pH changes from 4 to 5), it indicates the change in hydrogen ion concentration by 10 times. When there is change of pH by 2 units it indicate that the change in hydrogen ion concentration by 100 times. Due to this relationship, the pH scale is called as logarithmic.
While conducting many experiments, it is important to check the pH of any given solution. Conventionally, the pH is checked by pH paper or litmus paper. The change in litmus paper color indicates the change in pH, it is called as visual method. For precise noting of pH, an instrument is used and it is called as pH meter. The pH meter works on potentiometric principle.
As the acidic solution has more positive charge (H+ ion) than alkaline solution, and the acidic solution has greater potential to produce current than alkaline solution. The pH meter works by measuring the potential difference (voltage) of test solution and standard or known solution and calculate the pH. Hence, it works like a Voltmeter. To complete the circuit, two electrodes are connected in order to complete the circuit. The complete circuit allows the movement of electric current.
Working Principle of pH meter:
The working of pH meter is based on Nernst equation. Nernst equation derives the relation between the electric voltage and ion concentration. The Nernst equation derived for H+ ion concentration is the basis of pH meter. The working principle of pH meter is the potentiometry. The pH meter consists of glass (also called as indicator electrode) and reference electrode. The glass electrode consists of glass membrane, which is sensitive to hydrogen ion concentration of test sample solution. And the glass electrode potential varies from sample to sample. The reference electrode is standard and has constant potential. The reference electrode does not respond to test sample solution. The pH meter measures and compares the potential difference between both glass and reference electrodes.
Using the Nernst equation, the potential difference is used to measure the hydrogen ion concentration indicating the pH of given solution. Due to the potential difference between two electrodes, the electron flows and generates current. This generated current is measured by voltmeter. The relationship between the potential difference, generated current and pH has been derived. The potential difference of 1 pH is 59.16mV at 25° C and hence when there is difference of one pH unit, there will be change in voltage by 59.16mV. This relationship is employed in measuring the pH.
Construction of pH meter:
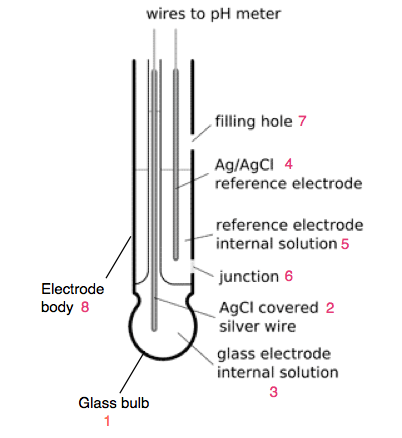
The pH probe of modern pH meter is a combined type, in which glass and reference electrode are placed into a rod like structure. The combined electrode consist of following parts-
http://www.ph-meter.info/pH-electrode-single-double-junction
- Glass bulb: It is a sensor that senses the H+ ion concentration and it is made from special type of glass and membrane. The glass bulb consists of 0.1M HCL.
- Internal electrode: It is the silver chloride electrode.
- Internal solution: The silver chloride electrode is dipped in buffer solution of 0.1 mol/L KCL of pH 7.
- Reference Electrode: It is also the silver chloride electrode.
- Internal Solution: The reference electrode is also dipped in buffer solution of 0.1 mol/L KCL of pH 7.
- Junction: It is made from ceramic junction also called as diaphragm that allows the contact of sample solution and reference electrolyte. It does not disturb the electric connection between both the electrodes.
- Filling hole: It is used for refilling the electrolyte.
- Electrode body: the body is from non-conductive glass or plastic.
The acidic solution is rich in H+ ion concentration. When pH probe is dipped in an acidic solution, the H+ ion moves close to the glass membrane of the sensitive glass bulb (external side of the bulb). Similar reaction occurs inside the bulb, which is filled with buffer solution of neutral pH. This neutral buffer solution has constant number of Hydrogen ions. The H+ ions present inside the bulb also moves close to glass membrane (internal side of the bulb). Hence, this causes the difference in the concentration of hydrogen ion or degree of hydrogen ion activity across the membrane causing difference in the potential (voltage). When the hydrogen ion concentration inside the glass bulb is less than the outside solution (test solution), then the given solution is acidic and hence the pH is lower than 7.
When the Hydrogen ions concentration across the membrane is same then it is called as neutral pH and the pH is equal to 7. If the concentration of hydrogen ion of inside the bulb than outside solution then the given solution is alkaline and the pH is more than 7. The pH meter measure the potential difference at both the electrodes and calculate the pH as per the Nernst equation.
MCQ Quize –
Results
#1. The water is neutral because
#2. The water contains…………… H+ ions
#3. When pH changes by 2 units, it indicates the change of pH by
#4. Why Silver chloride electrodes are used?
#5. Why KCL is used in pH meter?
References:
http://chemistry.elmhurst.edu/vchembook/184ph.html
https://www.ysi.com/ysi-blog/water-blogged-blog/2019/02/anatomy-of-ph-electrodes
http://www.ph-meter.info/pH-electrode-construction
https://www.explainthatstuff.com/how-ph-meters-work.html
Dr. Sangha Bijekar has 9 years of Teaching Experience at University level. She loves to get engage in teaching and learning process. She is into blogging from last two years. She intends to provide student friendly reading material. She is avid Dog Lover and animal rescuer. She is learned Bharatnatyam and Katthak Dancer. She is into biking and She also loves to cook.