HPLC (High Performance Liquid Chromatography) is one of the types of column chromatography that uses a pump to create high pressure. In traditional column chromatography, the mobile phase and sample drip or runs under gravity. But in HPLC, the sample runs with mobile phase under pressure through the column filled with stationary phase. The use of pump and pressure makes HPLC much more efficient than traditional chromatography.
In HPLC, the mobile phase is in liquid state (solvent or mixture of solvent) and stationary phase is in solid state (porous form). The basic principle and application of HPLC is similar to other chromatographic technique. HPLC is used for isolation, identification and quantification of sample. The detection of sample is more efficient and systematic due to the automation. The HPLC is widely used in Academia, Research and Industries.
Principle of HPLC-
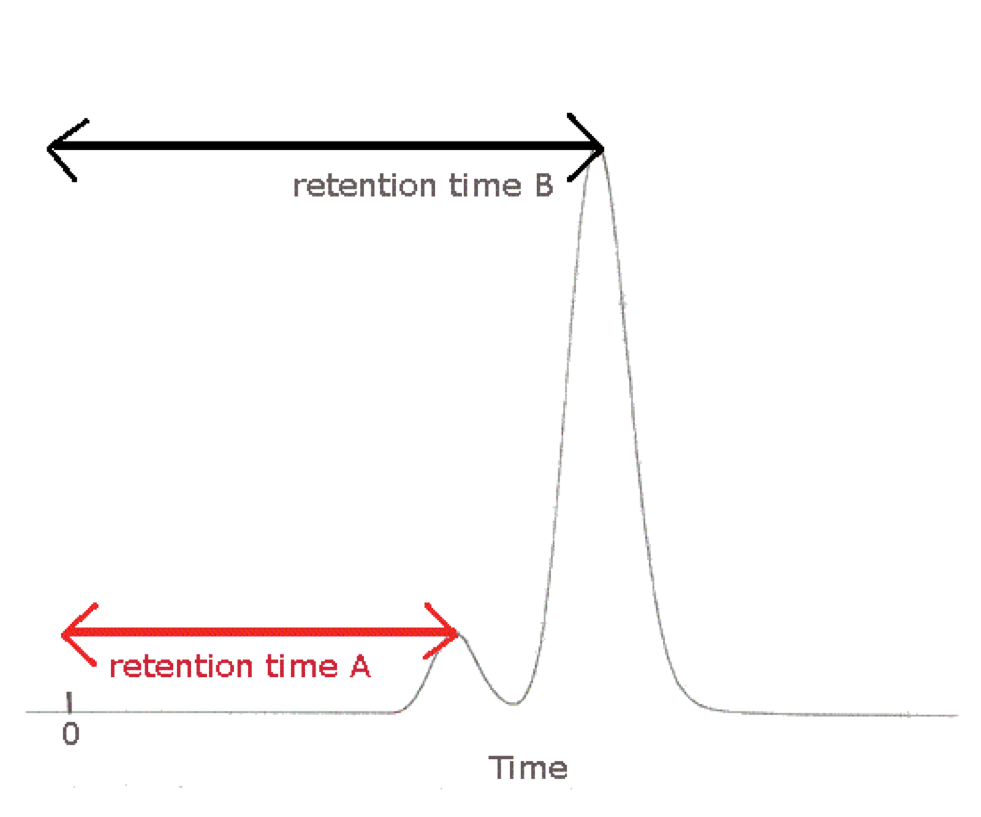
In HPLC, the separation and purification of mixture or sample is achieved because of having different affinity for stationary and mobile phase. The stationary phase is in granular state (porous particles) filled in the stainless steel column whereas the mobile phase is solvent or mixture of solvents. The injected sample runs with mobile phase and interacts differently with stationary and mobile phase. The time taken by compound to travel through column is called as retention time. The retention time varies from compound to compound and hence it is used as the characteristics for compound identification. The retention time is a duration taken by the sample to reach the detector. The signal received from detector is passed to HPLC software and it gives chromatogram.
Construction of HPLC –
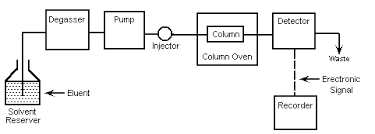
Solvent Reservoir –
In HPLC, the first component is solvent reservoir which stores the mobile phase with which sample runs in the column of HPLC.
Degasser –
The solvent or eluent may contains traces of gas such as oxygen. If gas remains present during analysis, the recorded detect it as noise and affect the quality of analysis. To remove the gas, the eluent is passed through a polymer tube which has small pores. These pores allow the gas to move out.
Pump –
- It is also called as solvent delivery system.
- The pump maintains the flow rate and pressure of the mobile phase.
- The stationary phase resist the mobile phase flow and hence higher pressure is needed for narrow columns.
- The pump is made from different mechanical parts like piston, drive, pump head, check valve, high-pressure pump unit, proportioning valve, mixer and degasser.
- The mobile phase is pressurized with the help of gas like nitrogen gas. The applied pressure determines the flow rate of mobile phase. To get appropriate result it is vital to maintain the constant pressure. Change in pressure may affect the retention time of the sample giving false results.
Injector –
- Role of the Injector is to inject the sample into HPLC.
- It is placed beside the HPLC pump.
- The automated injector injects the sample as per set schedule.
- Once the sample is injected it travels with mobile phase in the colum
Column-
- It is usually made from glass, plastic or stainless steel.
- The stationary phase is a polymeric gel or silica. The stationary phase interacts with sample with non covalent bond such as hydrogen bond, ionic interaction, hydrophobicity or hydrophilicity. The physical properties of stationary phase affect the retention time of the sample.
- The internal diameter also affects the elution profile of the sample.
- The column are either tightly packed with stationary phase or applied to the inner walls of the column.
- Temperature plays an important role for isolation, separation and quantification of sample. The column temperature needs to maintained and it is usually in the range of 50 to 80°C. This consistent temperature is created and maintained by column heater.
- There are different types of column available. The selection of column is based on the aim or motive of conducting the analysis. The different types of columns are as follows-
Normal Phase column – In such types of column, the stationary phase is polar (ex. Silica) whereas the mobile phase is non polar. Hence, the separation is based on the polarity of the components of mixture or sample. The component with higher polarity will interact more with stationary phase and hence it will retain in column for longer period of time.
Reverse Phase column – As per its name suggest, it is the reverse of normal phase hence the stationary phase is non -polar and mobile phase is polar. In such column, the polar molecule or component of mixture will get elute faster whereas non polar molecule with interact with stationary phase and it will retain in column for longer period of time.
Ion Exchange column – these columns are exclusively used for ionic or charged molecules separation. In such columns, the stationary phase contains charged or ionic molecules whereas the mobile phase is a buffer. This column works on the principle of opposite charges attract each other. If you are willing to separate negative ions from a mixture or sample, you need the column with positive ions. When you inject the sample, the desired negative molecules will interact electrostatically with positive ions in the stationary phase. Whereas the other molecules will elute and allowing us to separate the molecules based on their charge.
Size Exclusion column – This differentiate and separate the molecules based on the molecule size. For this, the column is filled with porous gel (polysaccharide) and silica as stationary phase. When sample or mixture of molecules of different size is injected in HPLC, the smaller molecules penetrate inside the pores of gel whereas the larger molecule cannot penetrate. Hence, the larger molecules are eluted first.
Detectors –
- The separated and eluted molecules need to detected and analyzed.
- The detectors help us to quantify the sample.
- The detectors mostly work by comparing the difference of eluted sample with mobile phase and only mobile phase.
- This difference is measured in the form of electronic signal and present in the form of chromatogram.
UV Detectors – The UV detector works on Beer Lamberts law. It states that absorption is directly proportional to the concentration of molecules. It means higher is the absorption, higher is the concentration of molecule. To produce UV light, deuterium lamp is used. The slit allows the selection of desired wavelength. The quantified sample is depicted in the form of chromatogram.
RI Detector– Refractive index is the measure of bending of ray of light when it passes from one medium to another. As sample and mobile phase have different refractive index, their difference is used to develop chromatogram. The RI detectors measure the change in the refractive index of sample and mobile phase and compare with only mobile phase (reference). The difference of RI is measured with the help of photodiode. The photodiode detect the RI difference and produces potential difference.
Recorder –
Recorder gives us the analyzed data in the readable format. The electronic signal received from the detector is not visible to us. The software associated with HPLC processed the electrical signal and create the readable data in the form of graph or chromatogram.
Applications of HPLC-
- For detection for impurities.
- To identify the molecules
- To quantify the molecules.
Here is a video on it –
References –
https://www.chemguide.co.uk/analysis/chromatography/.html
https://www.hnauer.net/en/System-Solutions/Analytical-Basics—principles-and-parameters
https://www.bio-rad.com/featured/en/columns.html
https://www.chromatographyonline.com/view/modern
Dr. Sangha Bijekar has 9 years of Teaching Experience at University level. She loves to get engage in teaching and learning process. She is into blogging from last two years. She intends to provide student friendly reading material. She is avid Dog Lover and animal rescuer. She is learned Bharatnatyam and Katthak Dancer. She is into biking and She also loves to cook.



I didn’t know that pump and pressure make HPLC more efficient. I think a ton of people just use the basic models. I would like to make it more efficient though.